Abstract
Introduction
Genome wide association studies (GWAS) have catalogued thousands of loci that influence blood traits, but genetic mechanisms and impacted cell types are often unknown. While some regulatory factors are blood cell-autonomous, other extrinsic regulatory factors respond to systemic physiology. Epidemiologic studies have correlated generalized obesity (increased body mass index, BMI) and high cholesterol with anemia, but cell types and mechanisms that mediate these effects are incompletely understood, and these studies do not provide evidence for causality. Thus, we wanted to use genetic effects of BMI and cholesterol on hemoglobin level (HGB) and other blood traits to infer causal relationships, identify relevant cell types, and propose genetic mechanisms.
Methods
Mendelian randomization (MR), akin to a "genetic randomized controlled trial", uses variants linked to an exposure trait to estimate causal effects of that exposure on a given outcome. 1 Random variant allele allocation at meiosis enables this approach to address confounding and reverse causality that can otherwise preclude causal inference from epidemiologic and cohort studies. Further, effects of multiple factors can be parsed using multivariable MR and causal mediation analyses to help explain genetic associations. 2 Our study used gender- and age-adjusted GWAS summary statistics from European individuals.
Results
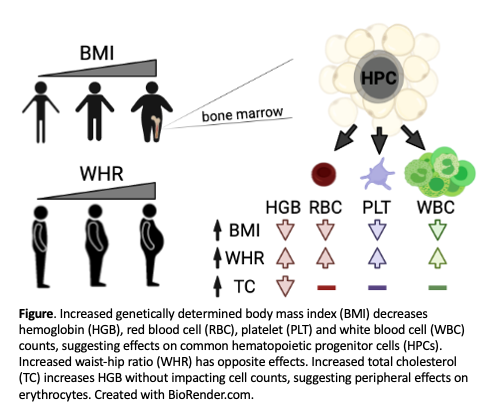
We hypothesized that increased genetically determined BMI would decrease HGB. Using a MR framework, we found that a 5 kg/m 2 (1 standard deviation unit) increase in BMI decreased hemoglobin by 0.06±0.01 g/dL (p=1x10 -5, Fig). Increased BMI also decreased erythrocyte count, and unexpectedly also decreased platelet and white blood cell counts (all with p<5x10 -3). These data showed that obesity-related mechanisms extended beyond the erythroid lineage, perhaps impacting multipotent hematopoietic progenitor cells (HPCs).
Similar to BMI, a 1 SD unit increase in total cholesterol decreased HGB (0.10±0.03 g/dL, p=2x10 -3, Fig). However, genetic effects of cholesterol were restricted to erythroid traits (HGB and hematocrit). Multivariable and mediation analyses confirmed that effects of BMI and cholesterol functioned through distinct genetic mechanisms.
We speculated that multilineage effects from BMI could reflect a genetic predisposition to accumulate bone marrow adipose tissue, which can impact HPCs and hematopoiesis. 3 A tendency for 'central' adiposity increases one's waist-hip ratio (WHR), and increases cardiovascular disease risk concordant with BMI. Unexpectedly, we found that increased WHR, in contrast to BMI, increased HGB (0.08±0.02 g/dL, p=9x10 -6) as well as erythrocyte, platelet, and white blood cell counts (all with p<4x10 -3, Fig). In multivariable experiments, the effects of WHR were exacerbated after accounting for BMI at the individual or population level. Thus, obesity impacts blood traits through genetically determined adipose distribution.
Conclusions
Our results confirm that BMI and cholesterol negatively impact HGB at a genetic level, consistent with clinical observations. The unexpected multilineage effects of genetically determined BMI most likely reflects a tendency to accumulate bone marrow adipose tissue, which in turn impacts HPCs and downstream blood cell production. Our findings suggest that adjustment for BMI and adiposity traits may be considered in blood trait GWAS analyses and illuminate opportunities to functionally dissect related genes and molecular pathways.
References
1. Hemani, G. et al. Elife 7, (2018). PMID: 29846171.
2. Sanderson, E. et al. Int. J. Epidemiol. 48, 713-727 (2019). PMID: 30535378.
3. Wang, H. et al. Front. Endocrinol.. 9, 694 (2018). PMID: 30546345.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal